The FDA has approved ZEVASKYN, an autologous gene-edited cell therapy, offering hope to patients with the debilitating skin disorder RDEB.
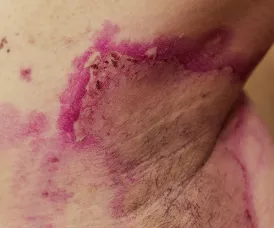
Recessive dystrophic epidermolysis bullosa (RDEB) is a rare genetic condition that causes extreme skin fragility, resulting in painful wounds and blisters. These wounds can cover large portions of the body, leading to severe physical and mental suffering. Patients with RDEB experience excruciating pain from even minimal contact, making life a constant battle. Traditional treatments focus on managing wounds, but the condition demands continuous, expensive care.
FDA Approval and Study Results
The FDA has approved ZEVASKYN, the first autologous gene-edited cell therapy for RDEB. This approval comes after positive results from the pivotal Phase 3 VIITAL study. A single application of ZEVASKYN showed that 81% of treated chronic wounds healed by 50% or more within six months, compared to just 16% of wounds treated with standard care. This groundbreaking therapy not only offers a long-lasting solution but also reduces the overall healthcare burden.
Impact on Patients’ Lives
Vishwas Seshadri, CEO of Abeona Therapeutics, emphasizes that ZEVASKYN offers more than just a medical breakthrough. It provides a life-changing opportunity for RDEB patients to heal and regain normalcy. “It’s about restoring dignity and reducing suffering,” Seshadri explains. For many families, the impact of ZEVASKYN has been described as a miracle, with one family noting that their child could sleep without pain for the first time in years.
The Journey of ZEVASKYN: From Concept to Approval
The development of ZEVASKYN began over a decade ago at Stanford University. Early studies showed the potential of introducing the COL7A1 gene into keratinocytes to treat RDEB. After the technology was licensed by Abeona in 2017, it took years to optimize the process for commercial use. Despite challenges and setbacks, including regulatory hurdles, the team’s perseverance led to successful Phase 3 trial results in 2022.
The Manufacturing Process
Creating ZEVASKYN involves an intricate process. A small biopsy is taken from patients, and the skin cells are genetically edited to express collagen VII, essential for skin integrity. These cells are cultured, tested, and formed into sheets that are then applied to the patient’s wounds in a precise surgical procedure. The treatment is unique in that it requires no long-term immune monitoring, unlike many other therapies.
Future Prospects and Applications
While ZEVASKYN has been developed for RDEB, there are potential applications for other conditions, including mitten deformities, a complication of RDEB. Additionally, Abeona is exploring the use of its AIM capsid library for other gene therapies targeting conditions such as retinal diseases and neurological disorders.
Abeona’s Vision for the Future
Looking ahead, Abeona plans to expand its gene therapy capabilities, leveraging the lessons learned from ZEVASKYN’s development to treat a broader range of conditions. With plans to introduce ZEVASKYN in 2025, the company is poised to revolutionize the treatment of RDEB and set new standards for gene therapies.
Related topics